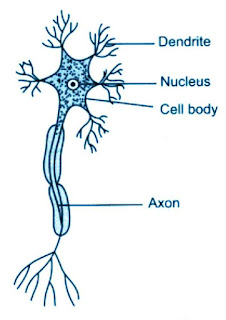
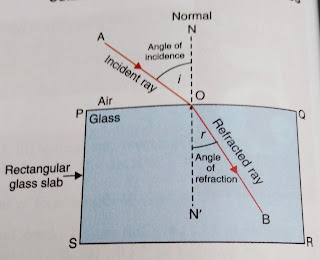
NCERT Book Periodic classification of elements // CBSE Class 10-Question Answer
NCERT BOOK PAGE 85
Question: Besides gallium, which other elements have since been discovered that were left by Mendeleev in his periodic table (any two)?
Answer: Scandium and germenium are the other two elements .
Question: What were the criteria used by Mendeleev in creating his periodic table?
Answer: The criteria used in periodic table by Mendeleev were-
i) elements are arrange in increasing order of their atomic number.
ii) on basis of similarity in chemical properties of the elements.
Question: Why do you think noble gases are placed in a separate group?
Answer: Noble gases have either stable duplate or octate electronic configuration, they do not react with any other elements.
Since all these noble gases are unreactive, have similar electronic configuration and similar behaviour, so they are placed in a seperate group that is zero group.
NCERT BOOK PAGE 85
Question : Use Mendeleev's periodic table to predict the formulae for the oxides of the following elements K, C , Al, Si and Ba.
Answer: Oxygen lies in VIA group. Therefore its valency is 2.
i) Potassium (K) lies in group IA. Therefore its valency is one. Therefore formula oxide of potassium is K2O.
ii) Carbon (C) lies in group IVA. Therefore its valency is 4.therefore oxide of carbon (C) is CO2.
iii) Aluminium (Al) lies in group IIIA . So valency of aluminium is 3. Therefore its formula of oxide is Al2O3.
iv) Silicon lies in group IVA. So its valency is 4. Therefore its formula of oxide is SiO2.
v) Since barium lies in group IIA so its valency is 2. Therefore oxide of barium is BaO.
More Related Search For You
Question: Besides gallium, which other elements have since been discovered that were left by Mendeleev in his periodic table (any two)?
Answer: Scandium and germenium are the other two elements .
Question: What were the criteria used by Mendeleev in creating his periodic table?
Answer: The criteria used in periodic table by Mendeleev were-
i) elements are arrange in increasing order of their atomic number.
ii) on basis of similarity in chemical properties of the elements.
Question: Why do you think noble gases are placed in a separate group?
Answer: Noble gases have either stable duplate or octate electronic configuration, they do not react with any other elements.
Since all these noble gases are unreactive, have similar electronic configuration and similar behaviour, so they are placed in a seperate group that is zero group.
NCERT BOOK PAGE 85
Question : Use Mendeleev's periodic table to predict the formulae for the oxides of the following elements K, C , Al, Si and Ba.
Answer: Oxygen lies in VIA group. Therefore its valency is 2.
i) Potassium (K) lies in group IA. Therefore its valency is one. Therefore formula oxide of potassium is K2O.
ii) Carbon (C) lies in group IVA. Therefore its valency is 4.therefore oxide of carbon (C) is CO2.
iii) Aluminium (Al) lies in group IIIA . So valency of aluminium is 3. Therefore its formula of oxide is Al2O3.
iv) Silicon lies in group IVA. So its valency is 4. Therefore its formula of oxide is SiO2.
v) Since barium lies in group IIA so its valency is 2. Therefore oxide of barium is BaO.