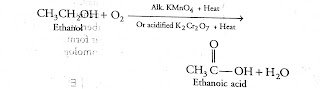NCERT Book Carbon and its Compounds Solution
NCERT BOOK Page No: 61 CBSE CLASS
Question (1) What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer : The electron dot structure for carbon dioxide is
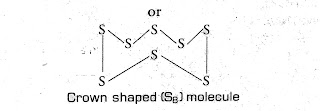
Ncert Question (2) What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint - the eight atoms of sulphur are joined together in the form of a ring.).
Answer:
Ncert Book: Page 68 and 69
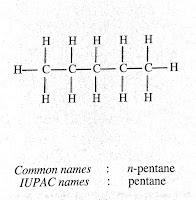
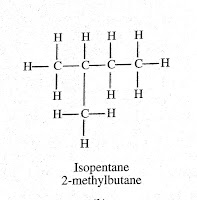
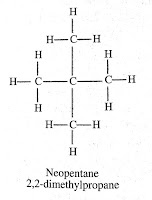
Question (1) . How many structural isomers can you draw for pentane?
Answer: We can draw three structural formula for pantane:
Question (2) What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer: The two properties are:
Catenation property
Tetravalency
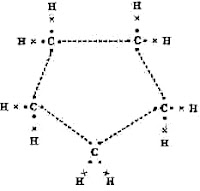
Question (3) What will be the formula and electron-dot structure of cyclopentane?
Answer: CnH2n is the general formula of cycloalkane. Here pentane means carbon numbers five.
Formula of cyclopentane = C5H10
Electron-dot structure of cyclopentane is
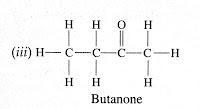
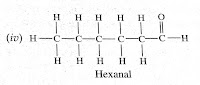
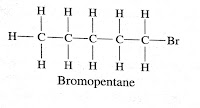
Question (4) Draw the structures for the following compounds.(i) Ethanoic acid (ii) Bromopentane (iii) Butanone (iv) Hexanal Are structural isomers possible for bromopentane?
Answer: Ethanoic acid
Bromopentane
Butanone
Hexanal
Yes, Bromopentane has three structural isomers. Depending upon where bromine is attached to carbon atom they are
1-bromopentane
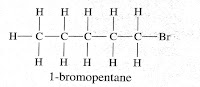
2-bromopentane
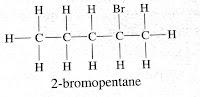
3-bromopentane
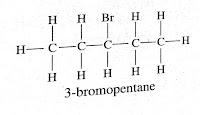
Question (5) How would you name the following compounds?
Answer: (i) Bromoethane
Methanal (formaldehyde)
(iii) Hexyne
Ncert Book Question Answer Solution (page 71)
Question (i) Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer: Oxidation is the removal of hydrogen or adding of oxygen to any compound. When ethanol convert into ethanoic acid, here two hydrogens remove from ethanol and add one oxygen atom. So it is an oxidation reaction.
Question (2) A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer: Welding need high temperature but when ethyne is burn in air it undergoes incomplete combustion. As a result it produce lesser amount of heat along with lots of smoke.
Whereas when ethyne and pure oxygen burnt then ethyne undergo complete combustion and produce lots of heat which is needed for welding. So we use mixture of ethyne and pure oxygen.
Question (1) What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer : The electron dot structure for carbon dioxide is
Ncert Question (2) What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint - the eight atoms of sulphur are joined together in the form of a ring.).
Answer:
Ncert Book: Page 68 and 69
Question (1) . How many structural isomers can you draw for pentane?
Answer: We can draw three structural formula for pantane:
Question (2) What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer: The two properties are:
Catenation property
Tetravalency
Question (3) What will be the formula and electron-dot structure of cyclopentane?
Answer: CnH2n is the general formula of cycloalkane. Here pentane means carbon numbers five.
Formula of cyclopentane = C5H10
Electron-dot structure of cyclopentane is
Question (4) Draw the structures for the following compounds.(i) Ethanoic acid (ii) Bromopentane (iii) Butanone (iv) Hexanal Are structural isomers possible for bromopentane?
Answer: Ethanoic acid
Bromopentane

Butanone
Hexanal
Yes, Bromopentane has three structural isomers. Depending upon where bromine is attached to carbon atom they are
1-bromopentane

2-bromopentane

3-bromopentane

Question (5) How would you name the following compounds?
Answer: (i) Bromoethane
Methanal (formaldehyde)
(iii) Hexyne
Ncert Book Question Answer Solution (page 71)
Question (i) Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer: Oxidation is the removal of hydrogen or adding of oxygen to any compound. When ethanol convert into ethanoic acid, here two hydrogens remove from ethanol and add one oxygen atom. So it is an oxidation reaction.
Question (2) A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer: Welding need high temperature but when ethyne is burn in air it undergoes incomplete combustion. As a result it produce lesser amount of heat along with lots of smoke.
Whereas when ethyne and pure oxygen burnt then ethyne undergo complete combustion and produce lots of heat which is needed for welding. So we use mixture of ethyne and pure oxygen.